Olefin Metathesis for biomass transformation
Key Words: Olefin metathesis, Ruthenium, Oleochemistry, Continuous Flow
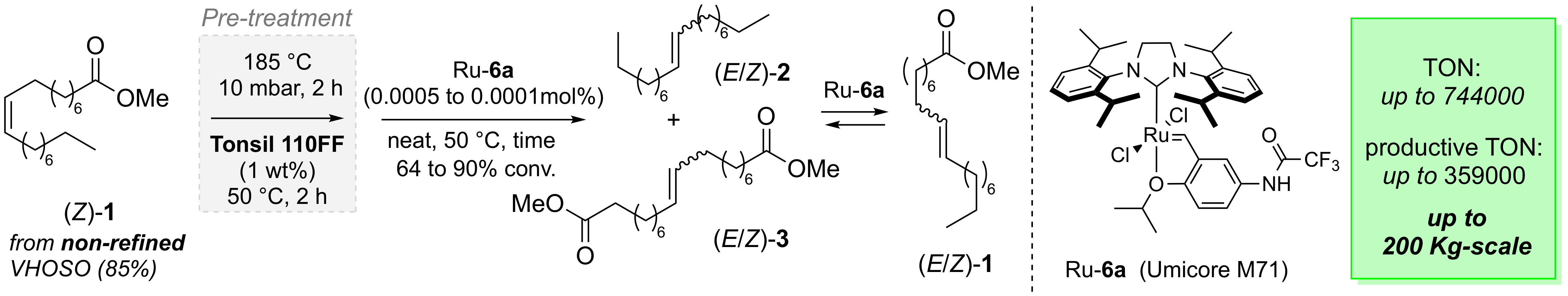
The production of bio-based building blocks from renewable raw materials has become one of the major challenges of the 21stcentury due to the inescapable rarefaction of fossil resources. To address this concern, olefin metathesis represents an efficient catalytic tool to convert olefins from biomass into molecules of interest, while guaranteeing a very low carbon footprint. In this context, we developed various eco-efficient and highly selective Ruthenium-based catalytic processes (including pilot scale) enabling to transform non-refined fatty esters, wood wastes and terpenes into valuable molecules such as pheromones, fragrances, monomers for materials or precursors of plasticizers and lubricants. Continuous flow processes are also being studied to ensure a more eco-efficient and safer production.
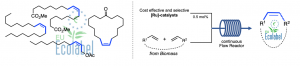
Contacts : Marc Mauduit, Christophe Crévisy, Sophie Rouen
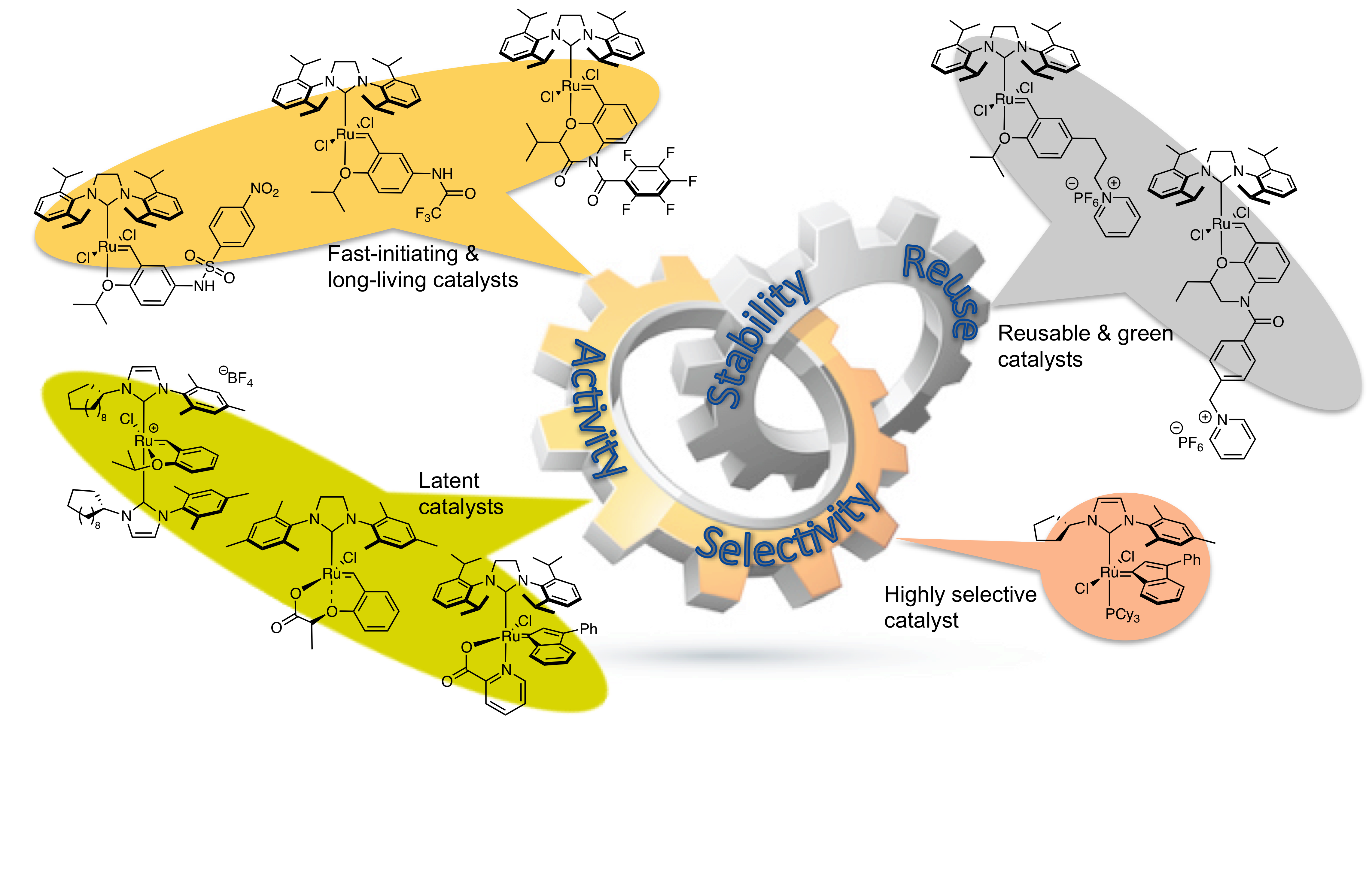
Design and synthesis of selective complexes for olefin and alkyne metathesis
Key Words: Olefin metathesis, Ruthenium, NHC, Z-selectivity
Olefin metathesis is one of the most powerful methodologies for the formation of C-C double bonds, with tremendous successes in numerous fields of application, including natural product synthesis, oleo-chemistry and material science. Driven by the discovery of new ruthenium catalysts with improved performances, our group is involved in the exploration of new types of complex architectures, with the objective of proposing innovative solutions addressing specific issues relating to sustainable chemistry. Thus, a library of eco-efficient and highly selective Ru-complexes was developed and successfully applied in various transformations, often industrially relevant. Alkyne metathesis catalyzed by Mo and W-complexes is also being studied to provide highly valuable polyynes.
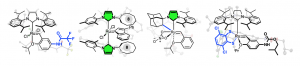
Contacts : Marc Mauduit, Christophe Crévisy, Sophie Rouen
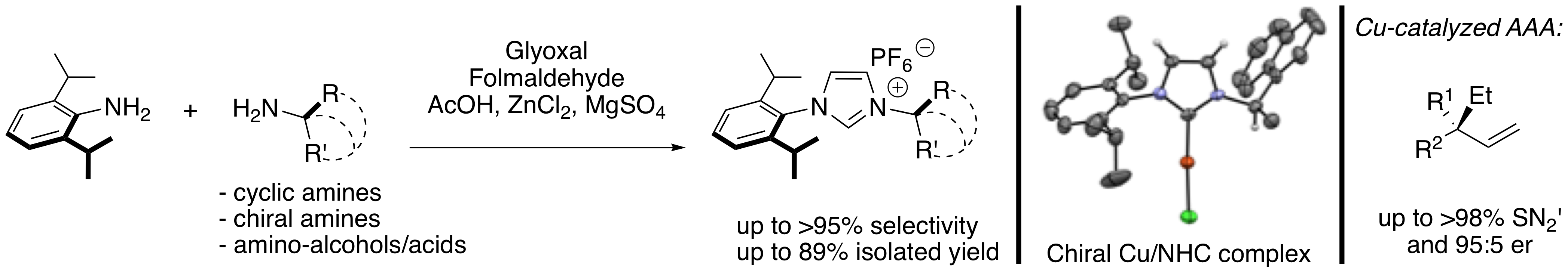
Design and synthesis of chiral NHCs and their related TM-complexes for enantioselective catalysis
Key Words: NHC, CAA(r)C, Transition metals, enantioselectivity
Due to their unique topology and a highly modular steric environment around the metal, chiral N-heterocyclic carbenes (NHCs) rapidly emerged as powerful stereo-directing ligands for a wide range of Transition Metal (TM)-catalyzed transformations. In this context, we designed the first chiral hydroxyalkyl-NHCs, which gave excellent enantioselectivities (up to 99% ee) in copper-catalyzed enantioselective alkylation involving Zn-, Mg-, and B-based reagents. Thus, numerous enantioenriched building-blocks were produced in high yields and used in the synthesis of natural molecules. Currently, we are involved in the development of original C1 and C2-symmetric TM-complexes from prochiral NHCs as well as from chiral Cyclic(Alkyl)(Amino)Carbenes (CAACs).

Contacts : Marc Mauduit, Christophe Crévisy, Sophie Rouen, Thomas Vives
Design and synthesis of new NHC-based ligands and evaluation in catalysis
Considering the great performances demonstrated by NHCs in catalysis, new NHC-based structures are currently under investigation with different electronic/steric properties and coordination modes. A special emphasis is made on the synthesis of earth-abundant transition metal complexes such as Iron, in order to discover new reactivity for reaction of high interest in industry as well as academia.
Contact : Marc Mauduit, Christophe Crévisy, Sophie Rouen, Thomas Vives
 |
Dr. Marc Mauduit (CV) |
Marc Mauduit obtained his Ph.D degree in 1999 from the University of Paris-Sud Orsay in France under the guidance of Prof. Y. Langlois. After working as postdoctoral fellow with Prof. S. Hanessian at Montreal University in Canada, he joined the Ecole Nationale Supérieure de Chimie de Rennes (UMR CNRS 6226 « Institut des Sciences Chimiques de Rennes ») in 2001 as Researcher Fellow at CNRS. In 2010, he was appointed Reseacher Director at CNRS. His research interest mainly focuses on organometallic chemistry, including olefin metathesis and enantioselective catalysis involving newly designed chiral ligands (N-heterocyclic carbenes and Phosphino-Schiff bases).
 |
Dr. Christophe Crévisy (CV) |
Christophe Crévisy received his PhD degree in 1992 from the University of Orléans. His PhD studies on the synthesis of antitumor enediynes were carried out under the direction of Dr. Jean-Marie Beau. From 1992 to 1993, he did a postdoctoral work with Pr. Pierre Deslongchamps at the University of Sherbrooke (Canada). Since 1994, he has been a senior lecturer at the Ecole Nationale Supérieure de Chimie de Rennes. He received his “Habilitation à Diriger les Recherches” degree in 2004. His main research interests are the development of new chiral ligands dedicated to asymmetric catalysis of reactions such as conjugated additions or allylic alkylation, the application of olefin metathesis to the valorisation of raw materials isolated from renewable resources and the development of new anti-tumor N-Heterocyclic Carbene/metal complexes.
 |
Dr. Sophie Colombel-Rouen (CV) sophie.rouen@ensc-rennes.fr |
Sophie Colombel-Rouen received her Ph.D. degree in 2012 from the National Institute of Applied Sciences (INSA) in Rouen under the supervision of Professor Xavier Pannecoucke and Dr. Eric Leclerc. Her Ph.D. studies were on the synthesis of difluorinated α-C- galactosylceramides and the assessment of their immunoregulation properties for the treatment of systemic autoimmune diseases. After postdoctoral work with Professor Thierry Benvegnu at Ecole Nationale Supérieure de Chimie de Rennes, she was appointed Engineer at ENSCR. Her main mission is to support the OMC (Organometallics, Materials & Catalysis) team in their research and to validate programs through organic synthesis.
 |
LIU Meng (Post-doctorant – 2024/2025) |
 |
ESCUREDO Julien (Post-doctorant – 2024/2026) |
 |
CASALTA Clément (Post-doctorant – 2024/2025) |
 |
BOUETARD Dylan (Thèse – 2021/2024) |
 |
MORVAN Fanny (Thèse – 2022/2025) |
 |
CHAILLOU Laura (Thèse – 2024/2028) |
 |
LE SAINT William (Ingénieur) |
 |
“Cyclic (amino)(barrelene)carbene Ru-complexes: synthesis and reactivity in olefin metathesis » J. Talcik, M. R. Serrato, A. Del Vecchio, S. Colombel-Rouen, J. Morvan, T.Roisnel, R. Jazzar, M. Melaimi, G. Bertrand, M. Mauduit, Dalton Trans., 2024, 5346. |
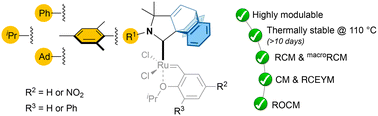 |
 |
“Tetracationic bis-triarylborane tetraynes as dual fluorescence and SERS sensors for DNA, RNA and proteins” D. P. Saftic, R. Ricker, P. Mentzel, J. Krebs, H. Amini, S. Lorenzen, N. Schopper, A. Kenđel, S. Miljanic, J. Morvan, M. Mauduit, Y. Trolez, I. Piantanida, T. B. Mardera, Microchemical Journal 2024 109665. |
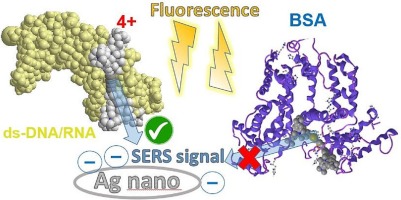 |
 |
“Challenges and Breakthroughs in Z-Enantioselective Olefin Metathesis” J. Morvan, A. Del Vecchio, J. Talcik, D. Bouëtard, M. Mauduit, Eur. J. Org. Chem. 2023, e202300671. |
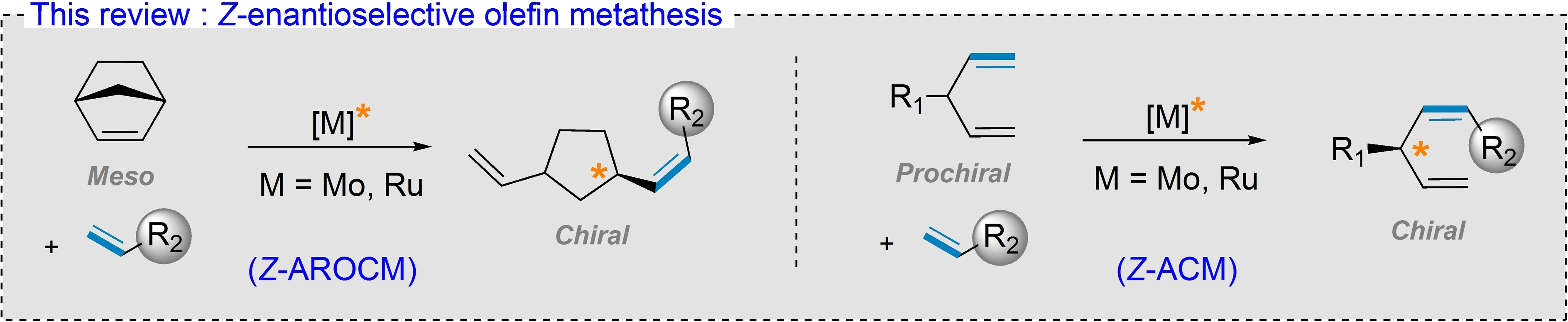 |
 |
“Circularly Polarized Luminescence from Cyclic (Alkyl)(Amino) Carbene Derived Propellers” J., Angew. Chem. 2023,135, e202305404. |
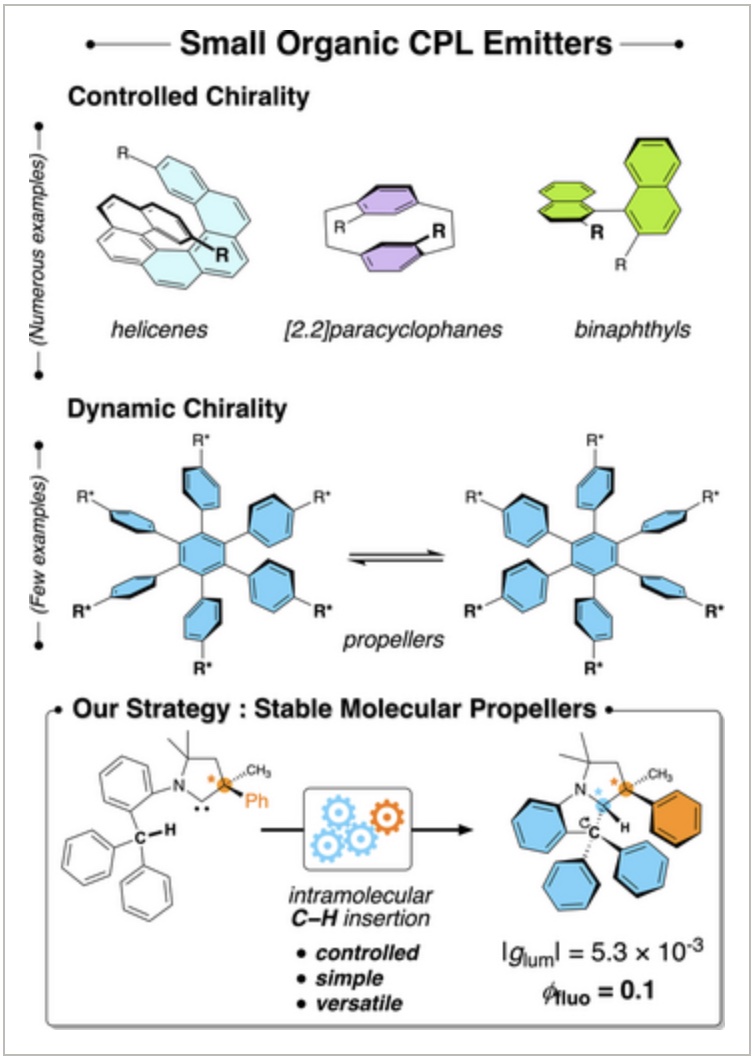 |
 |
“Highly Robust and Efficient Blechert-type Cyclic(alkyl)(amino)carbene Ruthenium Complexes for Olefin Metathesis” A. Del Vecchio, J. Talcik, S. Colombel-Rouen, J. Lorkowski, M. R. Serrato, T. Roisnel, N. Vanthuyne, G. Bertrand, R. Jazzar, M. Mauduit, ACS Catal. 2023, 13, 6195-6202. |
 |
 |
“Iridium(I) complexes with bidentate NHC ligands as catalysts for dehydrogenative directed C-H silylation” Chem. Commun. 2023, 59, 4193-4196. |
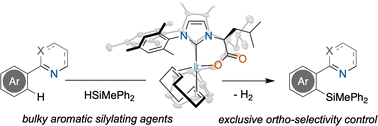 |
  |
“Chiral Atropisomeric-NHC Catechodithiolate Ruthenium Complexes for Z-Selective Asymmetric Ring-Opening Cross Metathesis of Exo-Norbornenes” Chem. Eur. J. 2023, e202300341 (early view). |
|
|
 |
“A Straightforward Access to Cyclic (Alkyl)(amino)carbene Copper (I) Complexes” Eur. J. Inorg. Chem., 2023, e202300074 (early view). |
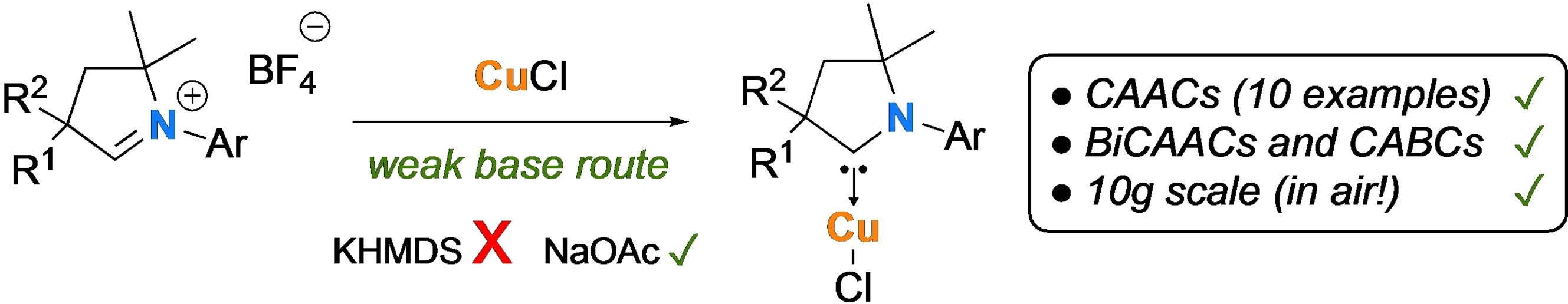 |
 |
“The Ambivalent Role of Rotamers in Cyclic(alkyl)(amino)carbene Ruthenium Complexes for Enantioselective Ring-Opening Cross-Metathesis” Organometallics 2023, 42, 495–504. |
 |
 |
“Cyclic(alkyl)(amino)carbene Ruthenium Complexes for Z-Stereoselective (Asymmetric) Olefin Metathesis” Catal. Sci. Technol. 2023, 13, 381-388. |
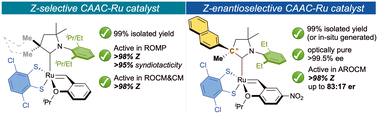 |
 |
“Ruthenium-NHC complex-catalyzed P(III)-directed C-H borylation of Arylphosphines” Chem. Commun. 2022, 58, 12082-12085. |
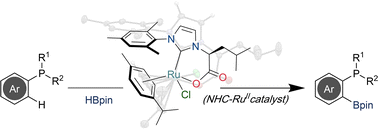 |
 |
“Challenges Arising from Continuous Flow Olefin Metathesis” Angew. Chem. Int. Ed. 2022, 61, e202209564. ✪ Highlighted in Org. Process Res. Dev. 2022, 26, 2997. |
 |
 |
“Titanium complexes with unsymmetrical imidazolin-2-iminato ligands” Dalton Trans. 2022, 51, 11448-11456. |
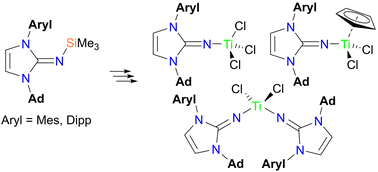 |
 |
“Chiral Oxazolidines Acting as Transient Hydroxyalkyl-Functionalized N-Heterocyclic Carbenes: An Efficient Route to Air Stable Copper and Gold Complexes for Asymmetric Catalysis” Chem. Sci., 2022, 13, 8773-8780. |
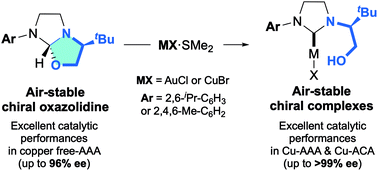 |
 |
“Metal-Catalyzed Metathesis of Fluorinated Alkenes: still a current major challenge » A. Nouaille, J. Lorkowski, X. Pannecoucke, M. Mauduit, T. Poisson, S. Couve-Bonnaire, ACS Catal. 2021, 11, 12307-12323. |
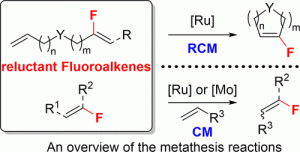 |
 |
“Catalytic Alkyne and Diyne Metathesis with Mixed Fluoroalkoxy-Siloxy Molybdenum Alkylidyne Complexes” , , , , , , Organometallics, 2021, 40, 2008-2015. |
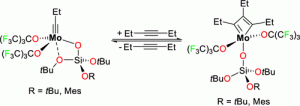 |
 |
“Continuous Flow Z-selective Olefin Metathesis: An Efficient Production of Macrocyclic Odorant Molecules and Pheromones” J. Morvan, T. McBride, I. Curbet, S. Colombel-Rouen, T. Roisnel, C. Crévisy, D. L. Browne, M. Mauduit. Angew. Chem. Int. Ed. 2021, 60, 19685-19690. (hot paper) |
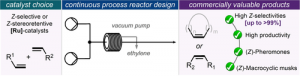 |
 |
“Hybrids of Cationic [4]Helicene and N-Heterocyclic Carbene as Ligands for Complexes Exhibiting (Chir)Optical Properties in the Far Red Spectral Window” R. Tarrieu, I. Hernandez Delgado, F. Zinna, V. Dorcet, S.Colombel- Rouen, C. Crévisy, O. Baslé, J. Bosson, J. Lacour, Chem. Comm. 2021, 57, 3793-3796. |
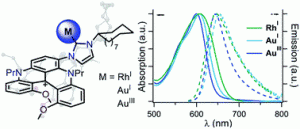 |
 |
“Cyclic(alkyl)(amino)carbene (CAAC) in Ruthenium Olefin Metathesis” J. Morvan, M. Mauduit, G. Bertrand, R. Jazzar, ACS Catal. 2021, 11, 1714-1748. |
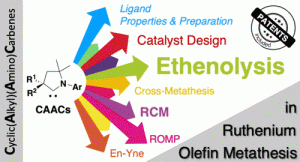 |
 |
“Optically pure C1-Symmetric Cyclic(alkyl)(amino)carbene (CAAC) Ruthenium-Complexes for Asymmetric Olefin Metathesis” J. Morvan, F. Vermesch, Z. Zhang, L. Falivene, T. Vives, V. Dorcet, T. Roisnel, C. Crévisy, L. Cavallo, N. Vanthuyne, G. Bertrand, R. Jazzar, M. Mauduit, J. Am. Chem. Soc. 2020, 142, 19895-19901. |
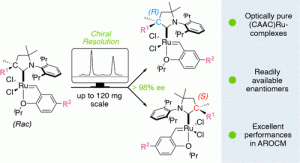 |
 |
“Expedient Synthesis of Conjugated Triynes via Alkyne Metathesis”, I. Curbet, S. Colombel-Rouen, R. Manguin, A. Clermont, A. Quelhas, D. S. Müller, T. Roisnel, O. Baslé, Y. Trolez, M. Mauduit, Chem. Sci., 2020, 11, 4934-4938. |
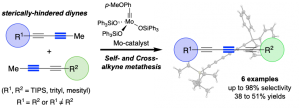 |
| “Copper-catalyzed enantioselective conjugate addition of organometallic reagents to challenging Michael acceptors.”, D. Pichon, J. Morvan, C. Crévisy, M. Mauduit, Beilstein J. Org. Chem. 2020, 16, 212–232. | |
 |
“DNA-Based Asymmetric Inverse Electron-Demand Hetero-Diels-Alder.” J. Mansot, J. Lauberteaux, A. Lebrun, M. Mauduit, J-J.Vasseur, R. Marcia de Figueiredo, S. Arseniyadis, J-M. Campagne, M. Smietana, Chem. Eur.J. 2020, 26, 3519 –3523. |
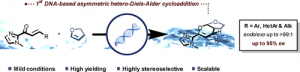 |
 |
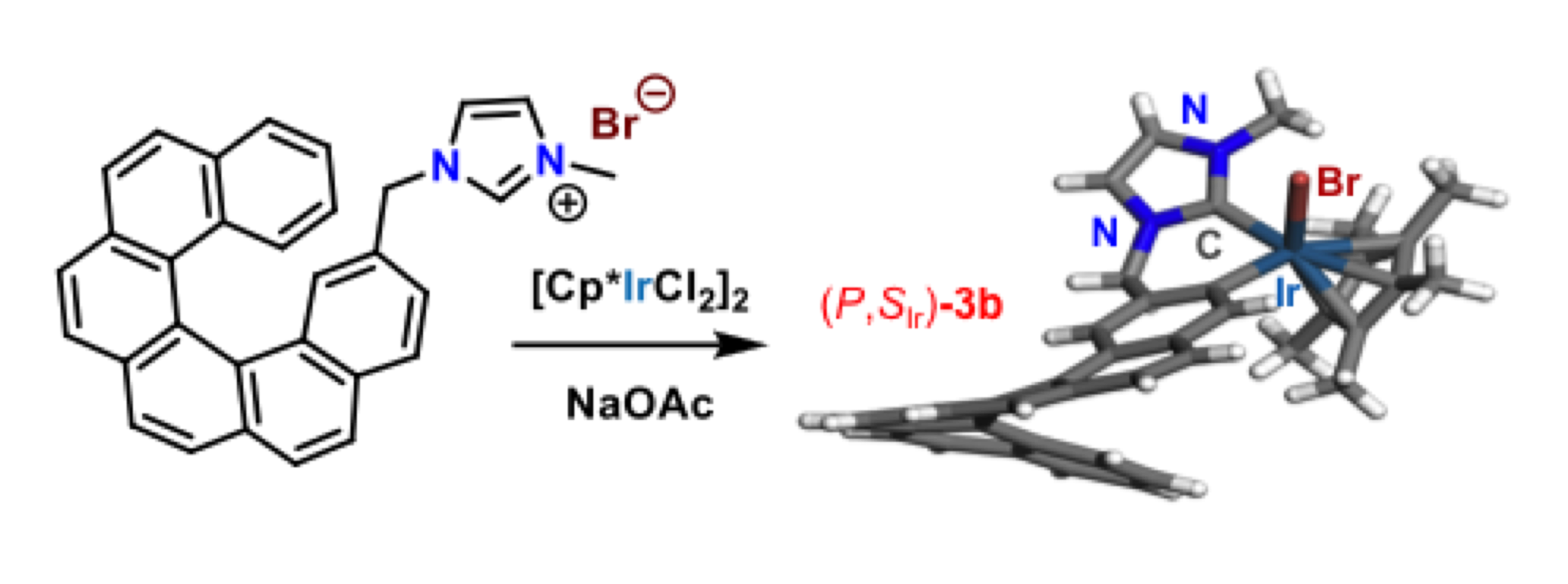
“From Prochiral N-Heterocyclic Carbenes to Optically Pure Metal Complexes: New Opportunities in Asymmetric Catalysis.” L. Kong, J. Morvan, D. Pichon, M. Jean, M. Albalat, T. Vives, S. Colombel-Rouen, M. Giorgi, V. Dorcet, T. Roisnel, C. Crévisy, D. Nuel, P. Nava, S. Humbel, N. Vanthuyne, M. Mauduit, H. Clavier, J. Am. Chem. Soc. 2020, 142, 1, 93–98. |
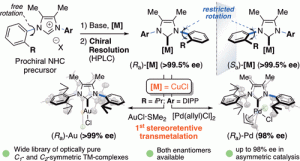 |
 |
“Iron-Catalyzed Enantioselective Intramolecular Inverse Electron-Demand Hetero Diels–Alder Reactions: An Access to Bicyclic Dihydropyran Derivatives.” J. Lauberteaux, A. Lebrun, A. van der Lee, M. Mauduit, R. Marcia de Figueiredo, J-M. Campagne, Org. Lett. 2019, 21, 10007−10012. |
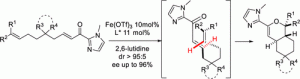 |
 |
“The debut of chiral cyclic (alkyl)(amino)carbenes (CAACs) in enantioselective catalysis.” D.Pichon, , Chem. Sci., 2019, 10, 7807–7811. |
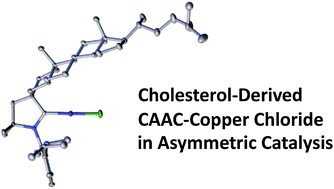 |
 |
“Activation of olefin metathesis complexes containing unsymmetrical unsaturated N-heterocyclic carbenes by copper and gold transmetalation.” F. Kamal, S. Colombel-Rouen, A. Dumas, J-P. Guégan, T. Roisnel, V. Dorcet, O. Baslé, M. Rouen and M. Mauduit, Chem.Commun., 2019, 55, 11583-11586 |
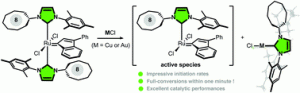 |
 |
“Visible Light Induced Rhodium(I)‐Catalyzed C−H Borylation.” J.Thongpaen, R. Manguin, V. Dorcet, T.Vives,C. Duhayon,M. Mauduit, O. Baslé, Angew.Chem. Int.Ed. 2019, 58,15244 –15248. |
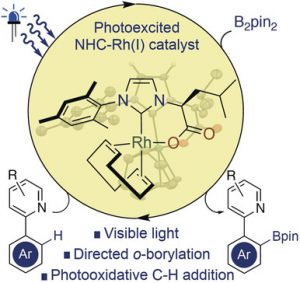 |
 |
“A kinetic resolution strategy for the synthesis of chiral octahedral NHC-iridium(iii) catalysts.” R. Manguin, D. Pichon, R. Tarrieu, T. Vives, T. Roisnel, V. Dorcet, C. Crévisy, K. Miqueu, L. Favereau, J. Crassous, M. Mauduit, O. Baslé, Chem. Commun., 2019, 55, 6058-6061. |
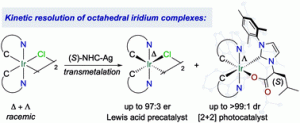 |
 |
“Highly selective macrocyclic ring-closing metathesis of terminal olefinsin non-chlorinated solvents at low dilution” A. Dumas, S. Colombel-Rouen, I. Curbet, G. Forcher, F. Tripoteau, F. Caijo, P. Queval, M. Rouen, O. Baslé, M. Mauduit, Catal. Sci. Technol. 2019, 9, 436–443. |
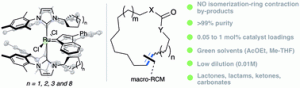 |
 |
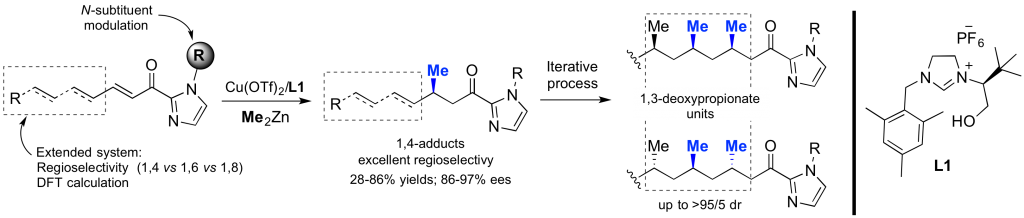
“Copper-Catalyzed Asymmetric Conjugate Additions of Bis(pinacolato)diboron and Dimethylzinc to Acyl- N-methylimidazole Michael Acceptors: A Highly Stereoselective Unified Strategy for 1,3,5,… n (OH, Me) Motif Synthesis.” J. Lauberteaux, C. Crévisy, O. Baslé, R. Marcia de Figueiredo, M. Mauduit, J-M. Campagne, Org. Lett. 2019, 21, 6, 1872–1876. |
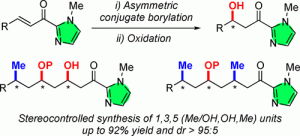 |
| “A tutorial review of stereoretentive olefin metathesis based on ruthenium dithiolate catalysts.” D. S. Müller, O. Baslé, M. Mauduit, Beilstein J. Org. Chem. 2018, 14, 2999–3010. | |
 |
 |
“Stereoretentive Olefin Metathesis Made Easy: In Situ Generation of Highly Selective Ruthenium Catalysts from Commercial Starting Materials.” D. S. Müller, I. Curbet, Y. Raoul, J. Le Nôtre, O. Baslé, M. Mauduit, Org. Lett. 2018, 20, 21, 6822–6826. |
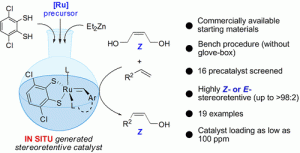 |
 |
“The Biomass Valorization by Olefin Metathesis: A Real Challenge for Tomorrow.” H. Olivier-Bourbigou, Y. Raoul, P. Piot, C. Crévisy, O. Baslé, M. Mauduit, Actualité Chimique 2018, 432, 18-28. |
 |
“Directed Ortho C-H Borylation Catalyzed Using Cp*Rh(III)-NHC Complexes.” J. Thongpaen, T. E. Schmid, L. Toupet, V. Dorcet, M. Mauduit, O. Baslé, Chem. Commun. 2018, 54, 8202-8205. |
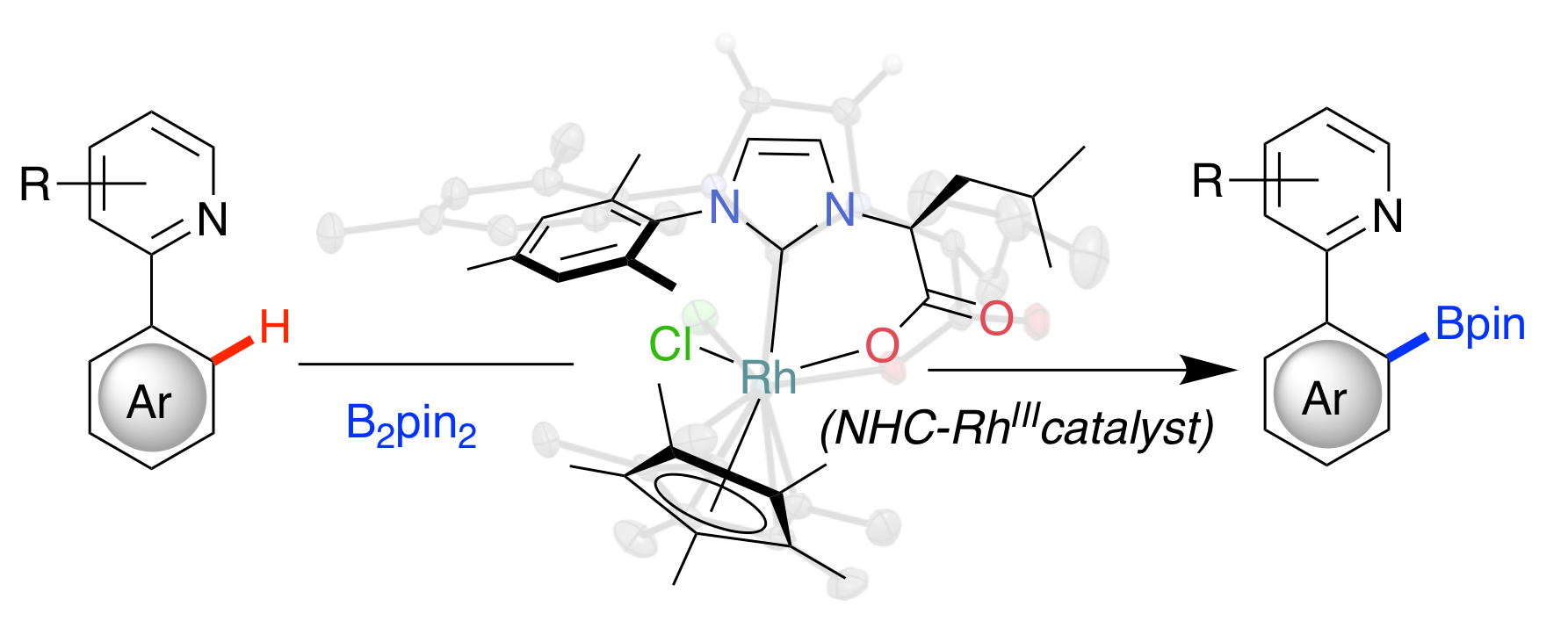 |
 |
“A Versatile and Highly Z-Selective Olefin Metathesis Ruthenium Catalyst Based on a Readily Acessible N-Heterocyclic Carbene.” A. Dumas, R. Tarrieu, T. Vives, T. Roisnel, V. Dorcet, O. Baslé, M. Mauduit. ACS. Catal. 2018, 8, 3257-3262. |
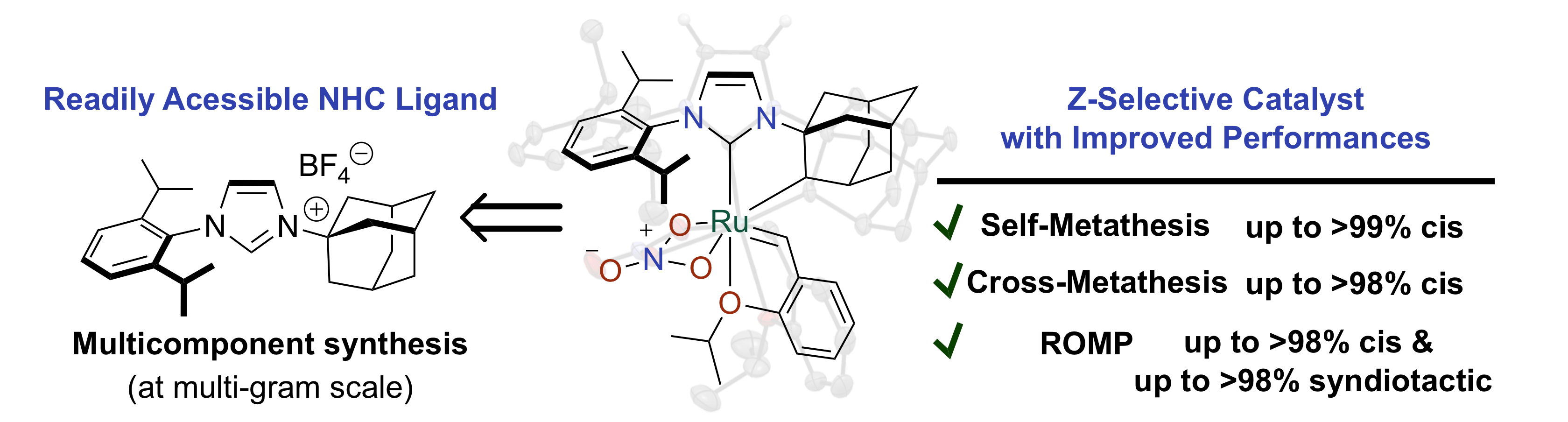 |
 |
“Synthesis and Application of Stereoretentive Ruthenium Catalyst on the Basis of the M7 and the Ru-Benzylidene-Oxazinone Design.” A. Dumas, D. S. Müller, I. Curbet, L. Toupet, M. Rouen, O. Baslé, M. Mauduit. Organometallics 2018, 37, 829-834. |
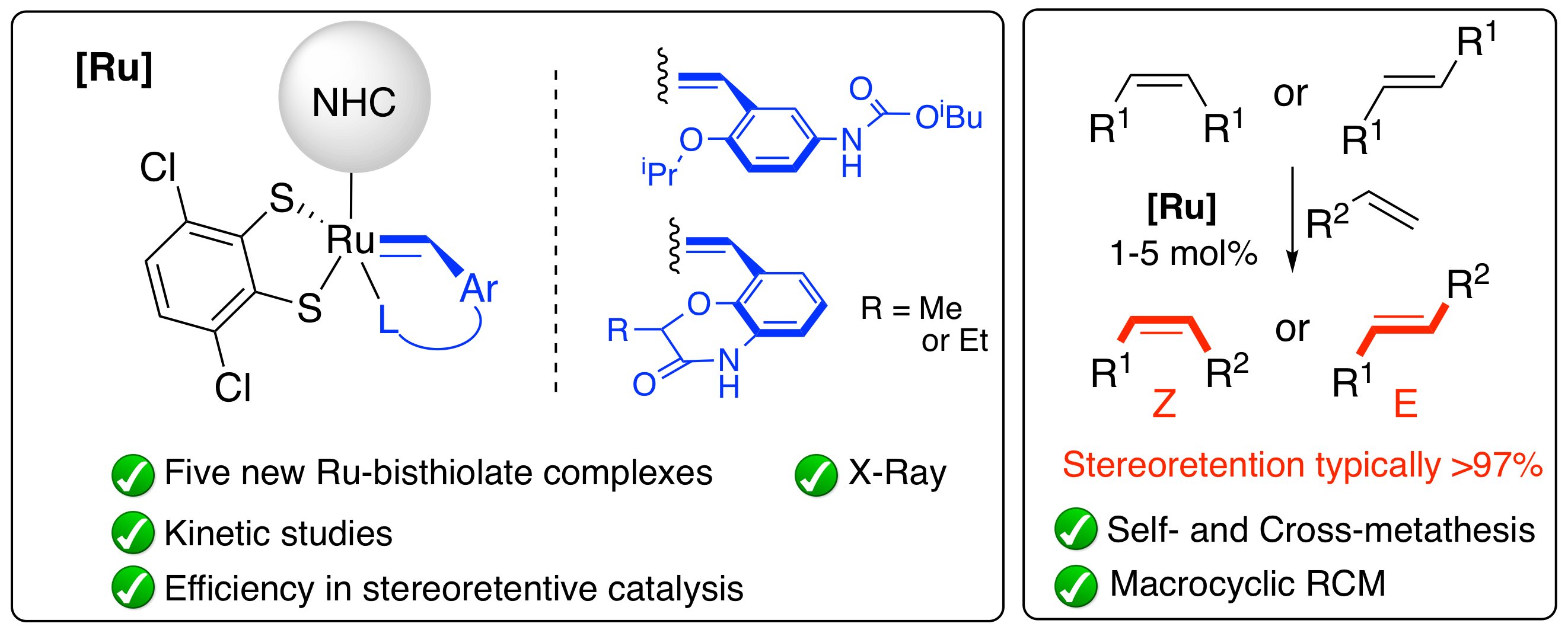 |
 |
“Bleaching Earths as Powerful Additives for Ru-Catalyzed Self-Metathesis of Non-Refined Methyl Oleate at Pilot Scale.” J. Allard, I. Curbet, G. Chollet, F. Tripoteau, S. Sambou, F. Caijo, Y. Raoul, C. Crévisy, O. Baslé, M. Mauduit. Chem. Eur. J. 2017, 23,12729-12734. |
 |
|
 |
“Readily Accessible Unsymmetrical Unsaturated 2,6-Diisopropylphenyl N-Heterocyclic Carbene Ligands. Applications in Enantioselective Catalysis.” R. Tarrieu, A. Dumas, J. Thongpaen, T. Vives, T. Roisnel, V. Dorcet, C. Crévisy, O. Baslé, M. Mauduit. J. Org. Chem. 2017, 82, 1880-1887. |
 |
 |
“From Environmentally Reusable Ionic-Tagged Ruthenium-Based Complexes to Industrially Relevant Homogeneous Catalysts: Toward a Sustainable Olefin Metathesis.” T. E. Shmid, A. Dumas, S. Colombel-Rouen, C. Crévisy, O. Baslé, M. Mauduit. Synlett. 2017, 28, 773-798 |
 |
 |
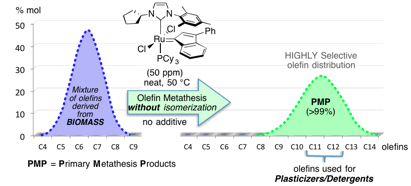
“Selective Metathesis of α-Olefins from Bio-Sourced Fischer–Tropsch Feeds.” M. Rouen, P. Queval, E. Borré, L. Falivene, A. Poater, M. Berthod, F. Hugues, L. Cavallo, O. Baslé, H. Olivier-Bourbigou, M. Mauduit. ACS catal. 2016, 6, 7970-7976 |
 |
 |
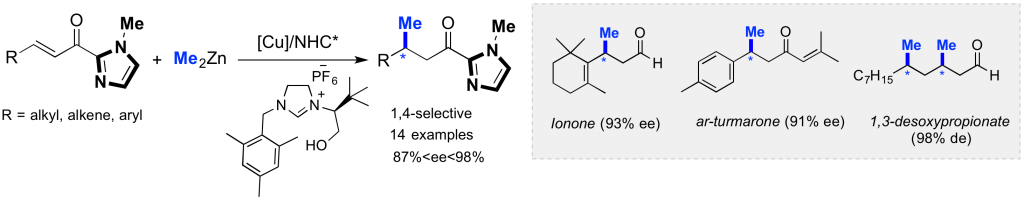
“Copper-Catalyzed Asymmetric Conjugate Addition of Dimethylzinc to Acyl-N-methylimidazole Michael Acceptors: Scope, Limitations and Iterative Reactions.” S. Drissi-Amraoui, T. E. Schmid, J. Lauberteaux, C. Crévisy, O. Baslé, R. Marcia de Figueiredo, S. Halbert, H. Gérard, M. Mauduit, J.-M. Campagne. Adv. Synth. Catal. 2016, 358, 2519-2540. |
 |
 |
“Electronic and chiroptical properties of chiral cycloiridiated complexes bearing helicenic NHC ligands.” N. Hellou, C. Jahier-Diallo, O. Baslé, M. Srebro-Hooper, L. Toupet, T. Roisnel, E. Caytan, C. Roussel, N. Vanthuyne, J. Autschbach, M. Mauduit, J. Crassous. Chem. Commun. 2016, 52, 9243-9246. |
 |
 |
“Copper-Catalyzed Asymmetric Conjugate Addition of Dimethylzinc to Acyl-N-methylimidazole Michael Acceptors : a Powerful Synthetic Platform.” S. Drissi-Amraoui, M. S. T. Morin, C. Crévisy, O. Baslé, R. Marcia de Figueiredo, M. Mauduit, J.-M. Campagne. Angew. Chem. Int. Ed. 2015, 54, 11830-11834. |
 |
 |
“Latent Ruthenium-Indenylidene Catalyst bearing a N-Heterocyclic Carbene and a Bidentate Picolinate.” T. Shmid, F. Modicom, A. Dumas, E. Borré L. Toupet, O.Baslé, M. Mauduit, Belstein J. Org. Chem. 2015, 11, 1541-1546. |
 |
 |
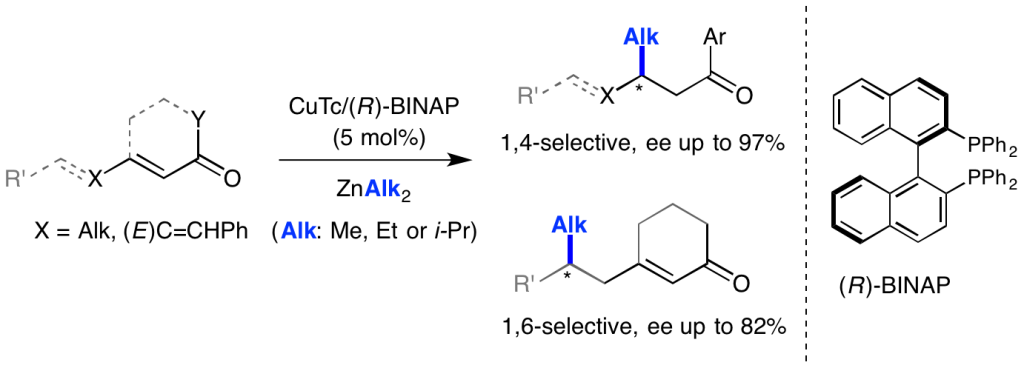
“Efficiency of Industrially Relevant Atropisomeric Diphosphines in Copper-Catalyzed 1,4-Asymmetric Conjugate Addition of Dialkylzincs to Cyclic or Acyclic Enones or Dienones” M. S. T. Morin, T. Vives, O. Baslé, C. Crévisy, V. Ratovelomanana-Vidal, M. Mauduit. Synthesis, 2015, 47, 2570-2577. |
 |
 |
“Multicomponent Synthesis of Chiral Bidentate Unsymmetrical Unsaturated N- Heterocyclic Carbenes: Copper-Catalyzed Asymmetric C-C Bond Formation.” C. Jahier-Diallo, M. S. T. Morin, P. Queval, M. Rouen, I. Artur, P. Querard, L. Toupet, C. Crévisy, O. Baslé, M. Mauduit. Chem. Eur. J. 2015, 21, 993-997. |
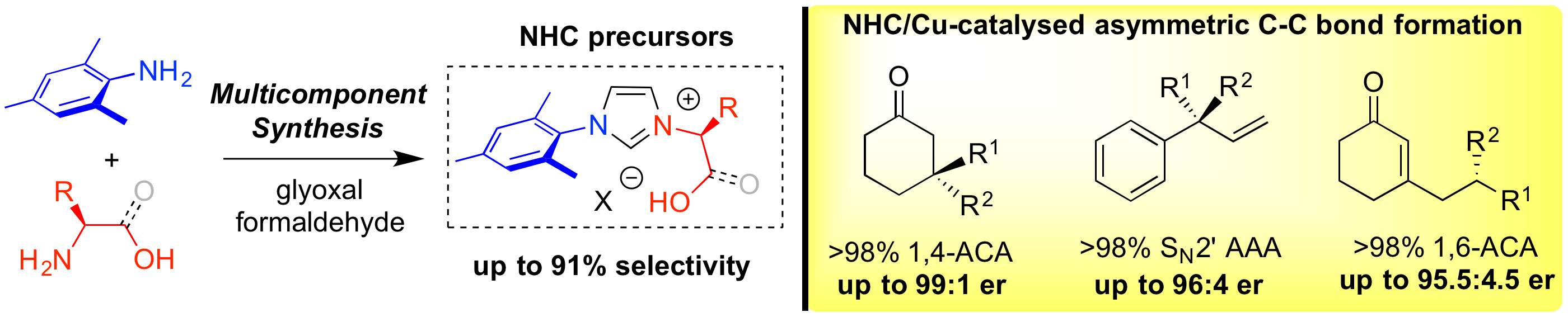 |
 |
“Cationic Bis N-Heterocyclic Carbene Ruthenium Complex: Structure and Application as Latent Catalyst in Olefin Metathesis.” M. Rouen, P. Queval, L. Falivene, J. Allard, L. Toupet, C. Crévisy, F. Caijo, O. Baslé, L. Cavallo, M. Mauduit. Chem. Eur. J. 2014, 20, 13716-13721. | 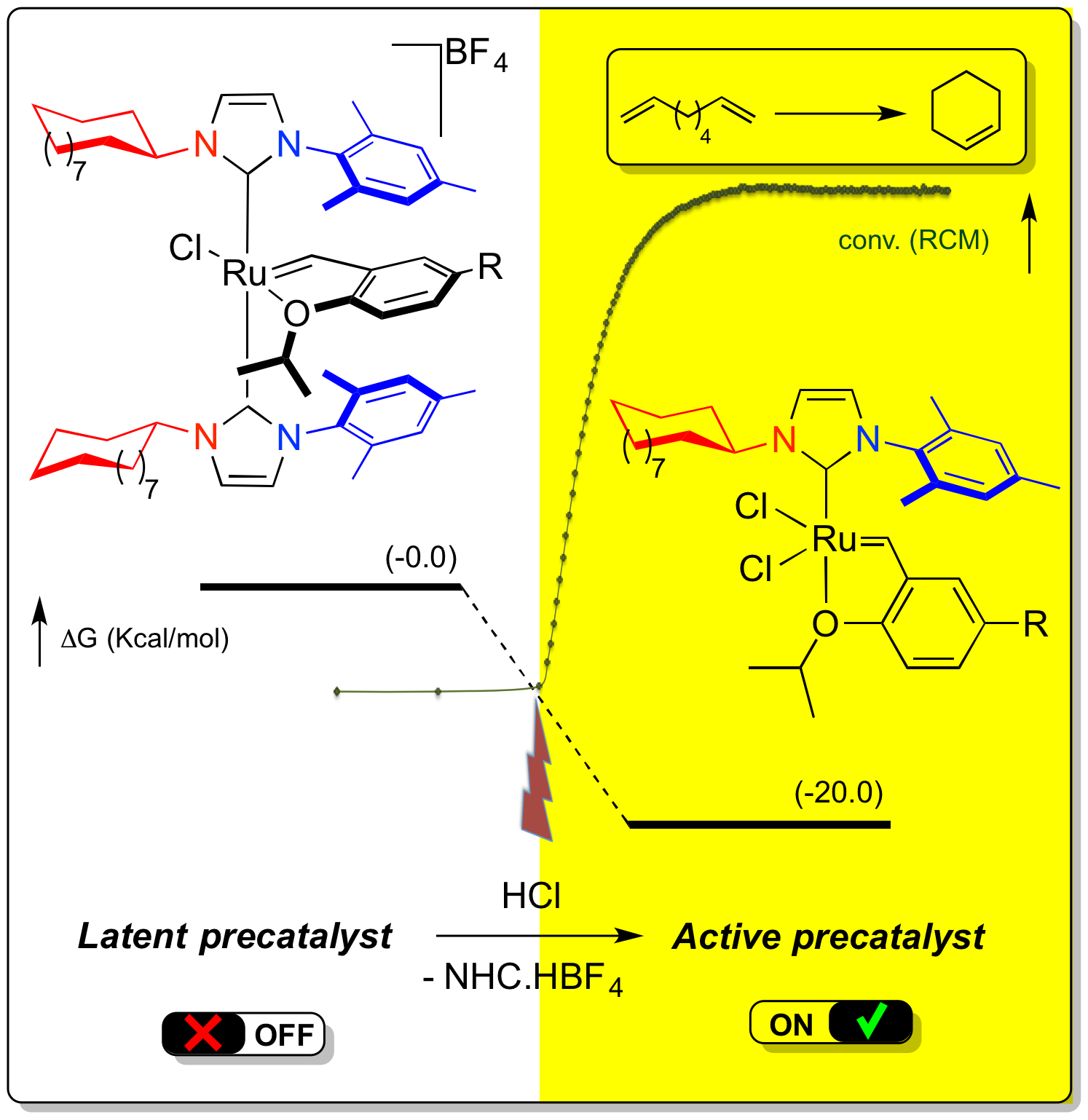 |
 |
“Multicomponent Synthesis of (a)chiral Unsymmetrical Unsaturated N-Heterocyclic Carbene (U2-NHC) Precursors; and Their Related Transition-Metal Complexes” P. Queval, C. Jahier, M. Rouen, I. Artur, J.-C. Legeay, L. Falivene, L. Toupet, C. Crévisy, L. Cavallo, O. Baslé, M. Mauduit. Angew. Chem. Int. Ed. 2013, 52, 14103-14107. |
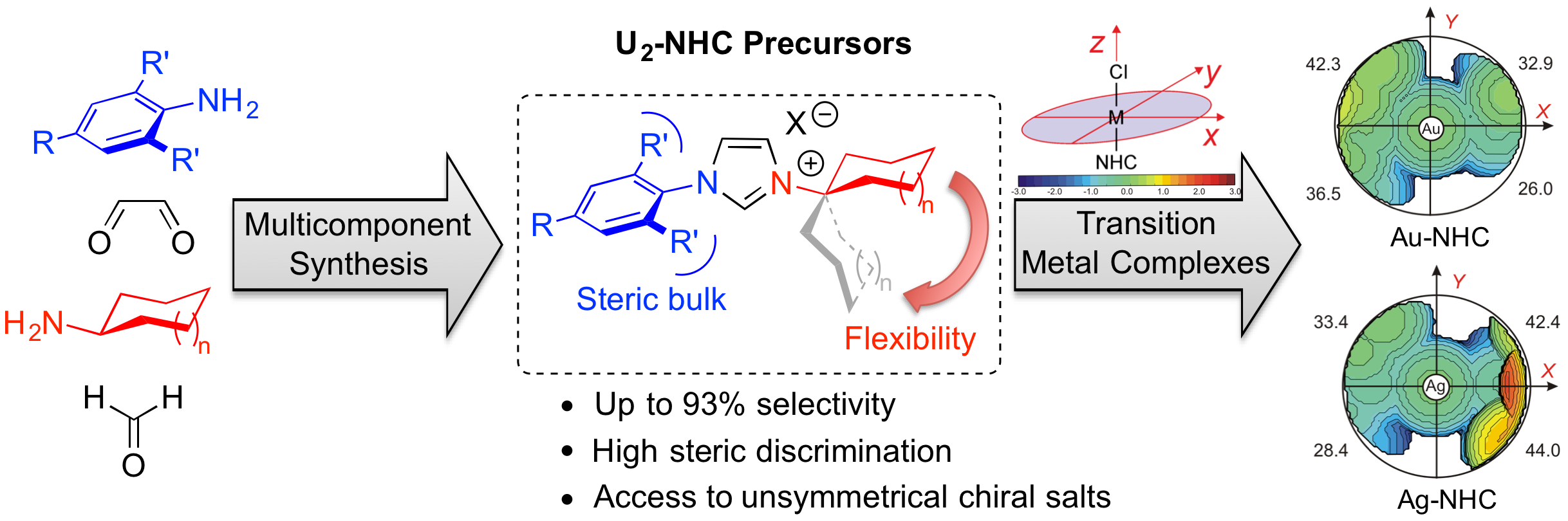 |
 |
« Enantioselective 1,6-Conjugate Addition of Dialkylzinc Reagents to Acyclic Dienones Catalyzed by Cu-DiPPAM Complex. Extension to asymmetric sequential 1,6/1,4 Conjugate Addition. » M. Magrez-Chiquet, M. S. T. Morin, J. Wencel-Delord, S. Drissi Amraoui, O. Baslé, A. Alexakis, C. Crévisy, M. Mauduit. Chem. Eur. J., 2013, 19, 13663. (Synfacts 2014, 10, 0042). |
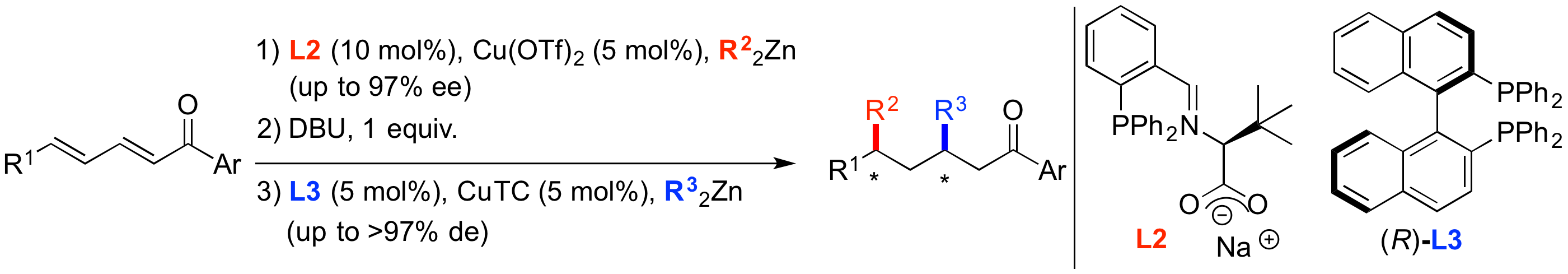 |
 |
« Synergic Effects Between N-Heterocyclic Carbene and Chelating-Benzylidene-Ether Ligands Towards the Initiation Step of Hoveyda-Grubbs Type Ru-Complexes. » D. J. Nelson, P. Queval, M. Rouen, M. Magrez, F. Caijo, E. Borré, I. Laurent, C. Crévisy, O. Baslé, M. Mauduit, J. M. Percy. ACS Catalysis, 2013, 3, 259-264 |
 |
 |
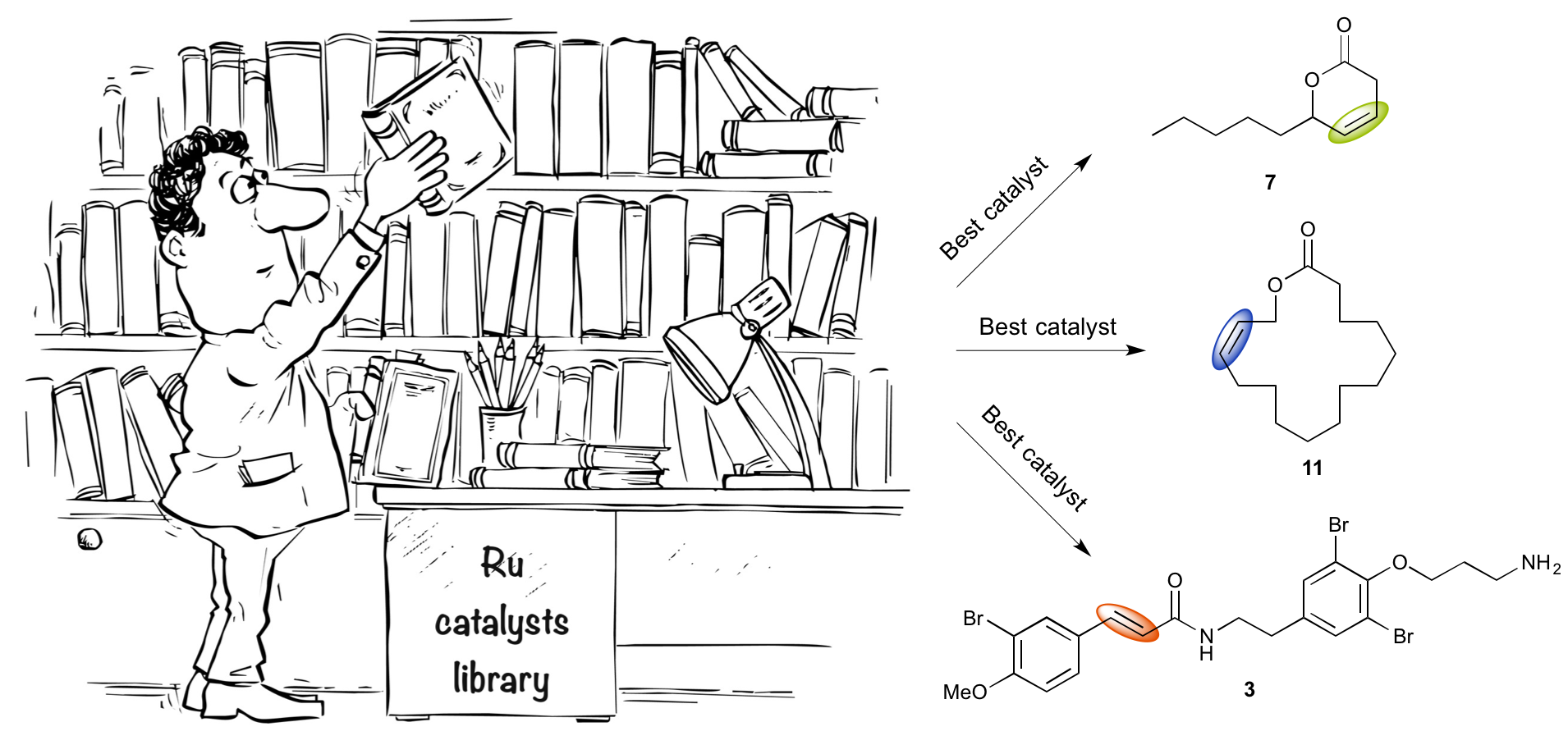
“Screening of a Selection of Commercially Available Homogeneous Ru-Catalysts in Valuable Olefin Metathesis Transformations.” F. Caijo, F. Tripoteau, A. Bellec, C. Crévisy, O. Baslé, M. Mauduit, O. Briel. Catal. Sci. Technol., 2013, 3, 429-435 |
 |
 |
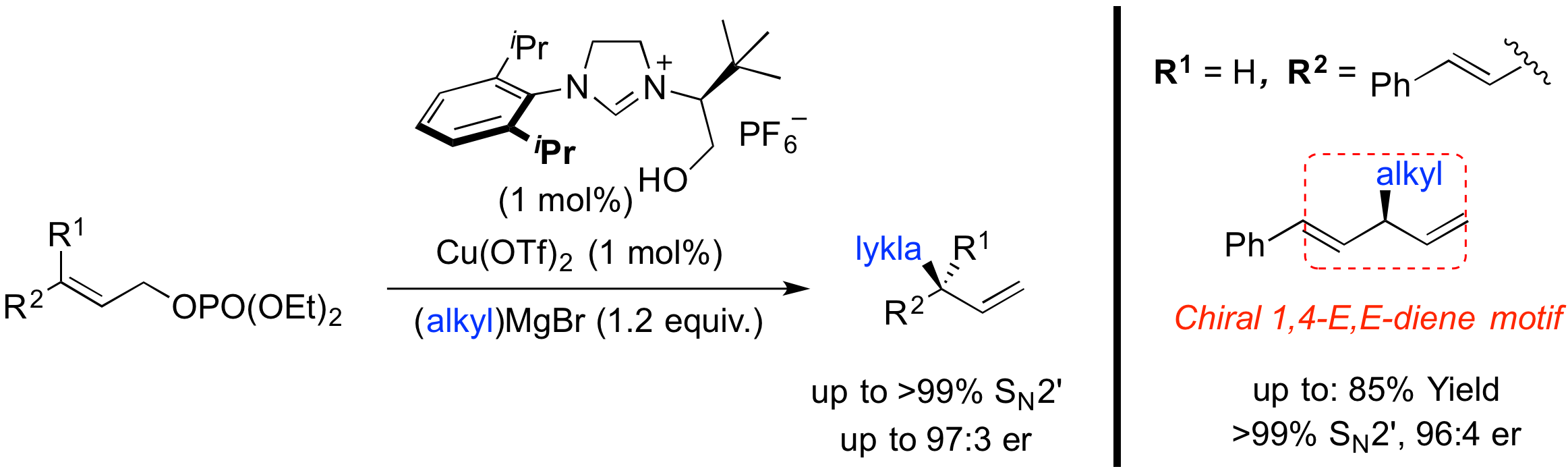
“Bidentate Hydroxyalkyl-NHC Ligands for Copper-Catalyzed Asymmetric Allylic Substitution of Allyl Phosphates with Grignard Reagents.” M. Magrez, Y. Le Guen, O. Baslé, C. Crévisy, M. Mauduit, Chem. Eur. J., 2013, 19, 1199-1203. (Synfacts 2013, 4, 0427) |
 |
 Students Office Students Office |

Meeting Room |
 Est Lab Est Lab |
|
 West Lab West Lab |
|




 GC/MS
GC/MS HPLC & GC
HPLC & GC